Waldenstrom Macroglobulinemia Market Sees Surging Demand Across the 7MM Amid BTK Inhibitor Advancements | DelveInsight
The Waldenstrom macroglobulinemia market is witnessing steady growth driven by advances in targeted therapies, particularly BTK inhibitors like IMBRUVICA and BRUKINSA. Rising awareness, improved diagnostic techniques, and increasing clinical trial activity are further propelling market expansion.
New York, USA, July 14, 2025 (GLOBE NEWSWIRE) -- Waldenstrom Macroglobulinemia Market Sees Surging Demand Across the 7MM Amid BTK Inhibitor Advancements | DelveInsight
The Waldenstrom macroglobulinemia market is witnessing steady growth driven by advances in targeted therapies, particularly BTK inhibitors like IMBRUVICA and BRUKINSA. Rising awareness, improved diagnostic techniques, and increasing clinical trial activity are further propelling market expansion.
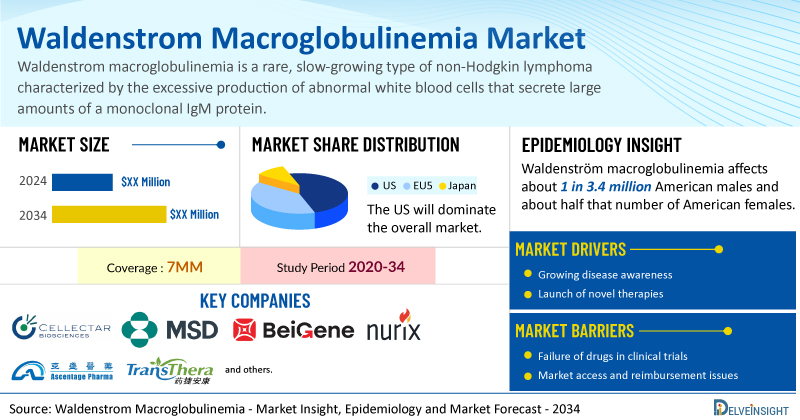
DelveInsight’s Waldenstrom Macroglobulinemia Market Insights report includes a comprehensive understanding of current treatment practices, emerging Waldenstrom macroglobulinemia drugs, market share of individual therapies, and current and forecasted Waldenstrom macroglobulinemia market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Waldenstrom Macroglobulinemia Market Report
- According to DelveInsight’s analysis, the total Waldenstrom macroglobulinemia market size is expected to grow positively by 2034.
- The United States accounts for the largest market size of Waldenstrom macroglobulinemia, in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan.
- Waldenström macroglobulinemia affects about 1 in 3.4 million American males and about half that number of American females. The incidence of waldenström macroglobulinemia is estimated to be about 5 per 1,000,000 people over the age of 50.
- Prominent companies, including Cellectar Biosciences, Merck Sharp & Dohme, BeiGene, Nurix Therapeutics, Ascentage Pharma, TransThera Biosciences, and others, are actively working on innovative Waldenstrom macroglobulinemia drugs.
- Some of the key Waldenstrom macroglobulinemia therapies in the pipeline include Iopofosine, Nemtabrutinib, Sonrotoclax, NX-5948, Lisaftoclax, TT-01488, and others. These novel Waldenstrom macroglobulinemia therapies are anticipated to enter the Waldenstrom macroglobulinemia market in the forecast period and are expected to change the market.
- In June 2025, Cellectar Biosciences announced that the US FDA granted Breakthrough Therapy Designation (BTD) for iopofosine I 131 in Waldenstrom macroglobulinemia.
- In June 2025, BeiGene showcased encouraging Phase I/II (CaDAnCe-101) results for BGB-16673 in waldenström macroglobulinemia at EHA 2025.
- In March 2025, the US FDA granted orphan drug designation to bexobrutideg (NX-5948) for the treatment of Waldenstrom macroglobulinemia.
Discover which Waldenstrom macroglobulinemia medications are expected to grab the market share @ Waldenstrom Macroglobulinemia Market Report
Waldenstrom Macroglobulinemia Overview
Waldenstrom macroglobulinemia is a rare, slow-growing type of non-Hodgkin lymphoma characterized by the excessive production of abnormal white blood cells that secrete large amounts of a monoclonal IgM protein. These abnormal cells accumulate in the bone marrow and can interfere with the normal production of blood cells.
The exact cause of Waldenstrom macroglobulinemia is not fully understood, but it is believed to arise due to genetic mutations, most commonly in the MYD88 gene, and possibly immune system dysregulation. Risk factors may include age, male gender, family history, and certain inherited conditions.
Symptoms of Waldenstrom macroglobulinemia can vary significantly and often develop gradually. Common clinical signs include fatigue, weight loss, night sweats, and swollen lymph nodes. The excess IgM protein can cause hyperviscosity syndrome, leading to headaches, vision problems, dizziness, and bleeding issues. Some patients may also experience neuropathy, cold sensitivity, or enlargement of the spleen and liver.
Diagnosis typically involves a combination of blood tests, bone marrow biopsy, and molecular testing to identify MYD88 or CXCR4 mutations. Imaging may also be used to assess organ involvement. Early and accurate diagnosis is key to managing this indolent but complex hematologic malignancy.
Waldenstrom Macroglobulinemia Epidemiology Segmentation
The Waldenstrom macroglobulinemia epidemiology section provides insights into the historical and current Waldenstrom macroglobulinemia patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The Waldenstrom macroglobulinemia market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Incident Cases of Waldenstrom Macroglobulinemia
- Gender-specific Cases of Waldenstrom Macroglobulinemia
- Age-specific Cases of Waldenstrom Macroglobulinemia
- Gene Mutations in Waldenstrom Macroglobulinemia
Download the report to understand which factors are driving Waldenstrom macroglobulinemia epidemiology trends @ Waldenstrom Macroglobulinemia Treatment Algorithm
Waldenstrom Macroglobulinemia Treatment Market
Significant progress has been made in the treatment of Waldenström macroglobulinemia, a rare form of cancer, with therapies increasingly tailored to its unique clinical challenges. The Waldenström macroglobulinemia treatment landscape is primarily led by BTK inhibitors such as IMBRUVICA and BRUKINSA, alongside rituximab-based regimens and BCL-2 inhibitors like VENETOCLAX, which serve as complementary options. These targeted therapies have revolutionized clinical outcomes by extending progression-free survival and enhancing response rates, while maintaining manageable side effects.
BRUKINSA (zanubrutinib) from BeiGene represents a next-generation BTK inhibitor, engineered for improved selectivity and reduced off-target activity compared to earlier BTK inhibitors. It received U.S. FDA approval in 2021 for treating adults with Waldenström macroglobulinemia and has also been authorized by the EMA and other global regulatory bodies for use in this and other B-cell malignancies.
IMBRUVICA (ibrutinib), developed by Janssen and AbbVie, was the first BTK inhibitor to gain FDA approval in 2015 for adult patients with Waldenström macroglobulinemia. As a pioneering agent in its class, it transformed the treatment approach by disrupting B-cell signaling pathways that drive malignant cell growth. That same year, the European Commission also approved its use.
Rituximab remains the most commonly used monoclonal antibody for treating Waldenström macroglobulinemia. It works by binding to the CD20 protein found on the surface of lymphoma cells, triggering their destruction. Administered via intravenous infusion in clinical settings, rituximab may be used as a standalone therapy or in combination with chemotherapy, targeted treatments, or other agents.
Learn more about the Waldenstrom macroglobulinemia treatment options @ Waldenstrom Macroglobulinemia Treatment Guidelines
Waldenstrom Macroglobulinemia Emerging Drugs and Companies
The potential drugs in the pipeline include Iopofosine (Cellectar Biosciences), Nemtabrutinib (Merck Sharp & Dohme), Sonrotoclax (BeiGene), NX-5948 (Nurix Therapeutics), Lisaftoclax (Ascentage Pharma), TT-01488 (TransThera Biosciences), and others.
Iopofosine is a small-molecule Phospholipid Drug Conjugate (PDC) engineered to deliver iodine-131 directly to cancer cells in a targeted manner. It was recently assessed in the pivotal Phase II CLOVER-WaM study involving patients with relapsed/refractory Waldenstrom macroglobulinemia (R/R WM). The U.S. FDA has granted Fast Track Designation to iopofosine for use in patients with lymphoplasmacytic lymphoma (LPL) and Waldenstrom macroglobulinemia who have undergone at least two prior therapies. Furthermore, the drug has received Orphan Drug Designation (ODD) for LPL/WM.
In the European Union, the European Commission has granted ODD for relapsed/refractory multiple myeloma and WM, along with PRIME designation specifically for WM, acknowledging its potential to address significant unmet clinical needs. In June 2025, the FDA also awarded Breakthrough Therapy Designation (BTD) to iopofosine I-131 for treating R/R WM.
Nemtabrutinib is an experimental, reversible, non-covalent BTK inhibitor under investigation for its ability to block oncogenic B-cell receptor signaling. It is active against both wild-type BTK and BTK pathway mutations. Ongoing Phase II trials are evaluating its effectiveness in hematologic cancers, including chronic lymphocytic leukemia (CLL) and Waldenstrom macroglobulinemia. Unlike conventional BTK inhibitors, nemtabrutinib retains activity even against BTK proteins that have developed mutations.
Sonrotoclax is a BH3 mimetic designed to inhibit BCL2, a protein that enables cancer cell survival. By mimicking natural signals that promote cell death, sonrotoclax induces apoptosis in B-cell malignancies. Preclinical and early clinical studies have demonstrated that it is a potent and selective BCL2 inhibitor, characterized by a short half-life and no accumulation in the body. It has shown encouraging clinical results across multiple B-cell cancers, with over 1,300 patients treated globally. The U.S. FDA has granted Fast Track Designation to sonrotoclax for treating Waldenstrom macroglobulinemia.
The anticipated launch of these emerging Waldenstrom macroglobulinemia therapies are poised to transform the Waldenstrom macroglobulinemia market landscape in the coming years. As these cutting-edge Waldenstrom macroglobulinemia therapies continue to mature and gain regulatory approval, they are expected to reshape the Waldenstrom macroglobulinemia market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for Waldenstrom macroglobulinemia, visit @ Waldenstrom Macroglobulinemia Management
Waldenstrom Macroglobulinemia Market Dynamics
The Waldenstrom macroglobulinemia market dynamics are anticipated to change in the coming years. The availability of targeted therapies such as BTK inhibitors (IMBRUVICA, BRUKINSA) and monoclonal antibodies (rituximab), along with the identification of MYD88 L265P and CXCR4 mutations, enables more personalized and effective treatment approaches, while emerging options like novel BTK inhibitors, CAR-T therapies, bispecific antibodies, and the development of new drug classes and combination regimens hold promise for significantly enhancing treatment outcomes.
Furthermore, many potential therapies are being investigated for the treatment of Waldenstrom macroglobulinemia, and it is safe to predict that the treatment space will significantly impact the Waldenstrom macroglobulinemia market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the Waldenstrom macroglobulinemia market in the 7MM.
However, several factors may impede the growth of the Waldenstrom macroglobulinemia market. Targeted therapies like BTK inhibitors are often expensive, creating accessibility and reimbursement challenges, especially in lower-income countries, while resistance or intolerance to existing treatments like IMBRUVICA and BRUKINSA further necessitate alternative options, yet stringent approval processes and high clinical trial costs can significantly delay the launch of these new therapies.
Moreover, Waldenstrom macroglobulinemia treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the Waldenstrom macroglobulinemia market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the Waldenstrom macroglobulinemia market growth.
| Waldenstrom Macroglobulinemia Report Metrics | Details |
| Study Period | 2020–2034 |
| Waldenstrom Macroglobulinemia Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Waldenstrom Macroglobulinemia Companies | Cellectar Biosciences, Merck Sharp & Dohme, BeiGene, Nurix Therapeutics, Ascentage Pharma, TransThera Biosciences, BioGene, Janssen, AbbVie, and others |
| Key Waldenstrom Macroglobulinemia Therapies | Iopofosine, Nemtabrutinib, Sonrotoclax, NX-5948, Lisaftoclax, TT-01488, BRUKINSA, IMBRUVICA, and others |
Scope of the Waldenstrom Macroglobulinemia Market Report
- Waldenstrom Macroglobulinemia Therapeutic Assessment: Waldenstrom Macroglobulinemia current marketed and emerging therapies
- Waldenstrom Macroglobulinemia Market Dynamics: Conjoint Analysis of Emerging Waldenstrom Macroglobulinemia Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Waldenstrom Macroglobulinemia Market Access and Reimbursement
Discover more about Waldenstrom macroglobulinemia drugs in development @ Waldenstrom Macroglobulinemia Clinical Trials
Table of Contents
| 1. | Waldenstrom Macroglobulinemia Market Key Insights |
| 2. | Waldenstrom Macroglobulinemia Market Report Introduction |
| 3. | Waldenstrom Macroglobulinemia Market Overview at a Glance |
| 4. | Waldenstrom Macroglobulinemia Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Waldenstrom Macroglobulinemia Treatment and Management |
| 7. | Waldenstrom Macroglobulinemia Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Waldenstrom Macroglobulinemia Marketed Drugs |
| 10. | Waldenstrom Macroglobulinemia Emerging Drugs |
| 11. | Seven Major Waldenstrom Macroglobulinemia Market Analysis |
| 12. | Waldenstrom Macroglobulinemia Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
Related Reports
Waldenstrom Macroglobulinemia Pipeline
Waldenstrom Macroglobulinemia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Waldenstrom macroglobulinemia companies, including BeiGene, Cellectar Biosciences, Inc., Nurix Therapeutics, Inc., VelosBio Inc., a subsidiary of Merck & Co., Inc. (Rahway, New Jersey USA), Schrodinger, Inc., Loxo Oncology, Inc., Nkarta, Inc., Merck Sharp & Dohme LLC, ADC Therapeutics S.A., Bio-Path Holdings, Inc., Millennium Pharmaceuticals, Inc., Carna Biosciences, Inc., among others.
Non-Hodgkin's Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NHL companies, including AbbVie, Genmab, Novartis, Angiocrine Bioscience, Autolus, Zentera Therapeutics, Jiangsu Hengrui Medicine, among others.
Non-Hodgkin's Lymphoma Pipeline
Non-Hodgkin's Lymphoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key NHL companies, including Novartis, AstraZeneca, Genentech, BioInvent, Genmab, SystImmune, Nordic Nanovector, Pacylex Pharmaceuticals, Artiva Biotherapeutics, Inc., Chipscreen Biosciences, Ltd., Timmune Biotech Inc., Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Gilead Sciences, Acerta Pharma BV, Adagene Inc, Conjupro Biotherapeutics, Inc., Rhizen Pharmaceuticals, Juventas Cell Therapy Ltd., Incyte Corporation, HUYA Bioscience International, SecuraBio, Genor Biopharma Co., Ltd., Kyowa Kirin Co., Ltd., Antengene Therapeutics Limited, Regeneron Pharmaceuticals, Jiangsu HengRui Medicine Co., Ltd., Xynomic Pharmaceuticals, Inc., BioTheryX, Inc., UWELL Biopharma, Kronos Bio, Bio-Thera Solutions, Spectrum Pharmaceuticals, Inc., Aptose Biosciences Inc., Miltenyi Biomedicine GmbH, Precision BioSciences, Inc., Teneobio, Inc., TCR2 Therapeutics, IGM Biosciences, Inc., among others.
Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key multiple myeloma companies, including Sanofi, Karyopharm Therapeutics, AbbVie, Takeda Pharmaceutical, Celgene, Bristol-Myers Squibb, RAPA Therapeutics, Pfizer, Array Biopharma, Cellectar Biosciences, BioLineRx, Celgene, Aduro Biotech, ExCellThera, Janssen Pharmaceutical, Precision BioSciences, Takeda, Glenmark (Ichnos Sciences SA), Poseida Therapeutics, Molecular Partners AG, Chipscreen Biosciences, AbbVie, Genentech (Roche), Janssen Biotech, Nanjing Legend Biotech, Merck Sharp & Dohme Corp., among others.
Diffuse Large B-cell Lymphoma Market
Diffuse Large B-cell Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key DLBCL companies, including Roche (Genentech), Biogen, Nektar Therapeutics, Merck, Allogene Therapeutics, Miltenyi Biomedicine, AstraZeneca, BioVaxys, ImmunoVaccine Technologies, Cellectar Biosciences, Galapagos, Novartis, Lyell, ImmPACT Bio, Pfizer, Kartos Therapeutics, 2seventy bio, Regeneron Pharmaceuticals, BeiGene, Ranok Therapeutics, Constellation Pharmaceuticals, Genmab, IDP Discovery Pharma S.L., Immunitas Therapeutics, Monte Rosa Therapeutics, SymBio Pharmaceuticals, AVM Biotechnology, Autolus Therapeutics, Kymera Therapeutics, Otsuka Pharmaceutical, Caribou Biosciences, Adicet Bio, Gilead Sciences, Xynomic Pharmaceuticals, Amgen, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
